Four oncology leaders share how AI has centralized care coordination for high-risk patients, leading to tremendous outcomes, both clinically and financially.
Despite notable progress in treatment options, cancer continues to rank as the second leading cause of death in the United States. While we’ve made significant strides in advancing cancer mortality rates, there is still considerable work ahead. One major contributing factor is the delayed diagnosis, nixing the opportunity to cure cancer while it’s still treatable.
Two proven initiatives that are often overlooked that can enhance early cancer detection are screening and incidental findings programs. Regrettably, these initiatives remain poorly adopted by both patients and health systems.
Part of the reason these programs are poorly adopted in health systems is a lack of clear ownership. Screening and incidental findings programs require a lot of coordination, across many service lines, and there often isn’t one central team responsible for oversight. Additionally, tracking these patients can be incredibly laborious and time consuming.
To better understand how AI is powering stage shift in cancer diagnosis, I sat down with four oncology leaders–two clinicians and two administrators–to see how they’ve spearheaded incidental findings programs at their respective health systems.
While they each share unique perspectives, one thing is for sure, innovative oncology leaders recognize the importance of owning and funding early detection programs. Diverging from the traditional focus of only caring for post-diagnosis patients, these oncology leaders demonstrate how early intervention from oncology teams can save millions of lives and grow cancer services.
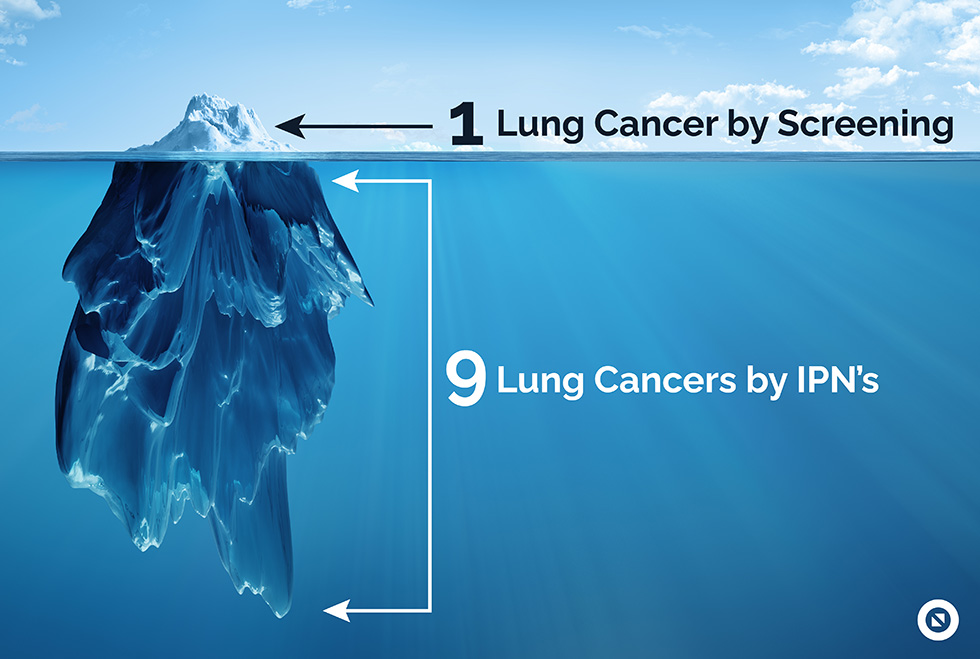
Incidental findings are abnormalities found by an imaging test that is unrelated to the reason the test was ordered. Nationally, 40% of all scans performed surface an incidental finding but 70% of these will not receive timely and appropriate follow-up.
Aki Alzubaidi, MD: Why should health systems invest in incidental findings programs?
Linda Lee, System VP, Cancer Care Services LCMC Health: The patient benefits the most because through these programs, we’re able to diagnose cancer sooner and in some cases, that means to cure cancer. Most hospitals have a heart for their communities. To be able to dial back the stage at time of diagnosis is such a commitment to a community to be able to conquer cancer; and it’s such a reduction of burden on the healthcare system than when you’re trying to care for somebody that’s an end-stage for lung cancer, which is terminal. So I think that the business case for anybody considering this is that not only does the patient benefit first and foremost, but all the disciplines that touch lung cancer or diagnostic nodules, you’re looking at radiologists, pulmonologists, interventional pulmonologists, radiation oncologists, medical oncologists, surgeons, thoracic surgeons. There’s something to be gained for them to be invested in a program. If you have a nodule committee or nodule board, everybody there is going to get referrals. So that’s easy to justify from the financial point of view.

Michael Gieske, MD, Director of Lung Cancer Screening, St. Elizabeth: Well, in Kentucky, we’re really ground zero in the fight against lung cancer. We have a very target-rich audience here, unfortunately, and we have the highest mortality, the highest incidence. We don’t do very well with five-year survival rates either. And the key is, especially as we get further into population health, is to try to catch cancer early, when it’s much cheaper to treat than when you catch it in the later stages. And that’s one of the messages that, we’re trying to get across.
It’s not only the right thing to do, it can be revenue positive as well, especially as we get further along the lines into value-based medicine.
Alzubaidi, MD: It’s clear that this is the right thing to do for patients but one of the key challenges is the ownership. Majority of health systems lack an “Incidental Findings” or “Screenings” service line. But in working with over 50 health systems with these programs, we’ve noticed that a lot of these programs end up being managed by Oncology or Cancer Services. Tell me, why you think oncology should be the ones, versus another department, to champion these programs.
Bridget LeGrazie, AVP, Oncology, Virtua Health: Well, in my opinion, we in Oncology understand the complexities of cancer diagnosis, the treatment planning involved, and the patient experience. We are the ones that are receiving the patient at the time of diagnosis and from a patient experience perspective, those days, those thousands of endless minutes for a patient waiting to find out whether their incidental nodule needs action or do they have cancer. Quite often it’s the oncology team that is being asked those questions by patients. Our nurse navigators get calls all the time, ‘hey, this was found, what do I do next, what if?’ So I think with our oncology nurse navigators are the standard setter for us in bringing patients through the cancer journey. Why don’t we expand that and place the nurse navigator higher up in the journey? At Virtua, we have a high-risk nurse navigator, we have a pulmonary clinic nurse, and we have an oncology nurse navigator. And we all work very well together to make that the best experience possible.
And then from a business standpoint, as a service line administrator for oncology, I’m the one who’s being looked to to say, ‘what’s the forecast for thoracic surgery? What’s the forecast for interventional pulmonology? Is there going to be growth in that service line?’ And the answer is yes.
We’ve seen significant downstream opportunities for revenue and ROI from our screening program and incidental findings program. But I think most importantly, it’s the right thing to do.
So when you marry the two together, it’s oncology, in my opinion, where it makes a lot of sense.
Alzubaidi, MD: “What has the downstream impact of introducing an AI solution to support your cancer screening and incidental findings program been like at your facility?”
Russell Langan, MD, FACS, FSSO, Associate Chief Surgical Officer, System Integration and Quality & Director of Surgical Oncology, Northern Region, RWJBarnabas Health: So what we have found in moving from the traditional antiquated way of seeing a patient and putting them on an Excel spreadsheet is, one, an immediate increase in quality, of course, because the way the platform runs is twofold. You have the identification of a patient that pulls a patient in, and then you have the longitudinal surveillance platform that’s now cloud-based and electronic and auto-populates pancreatic, gland data, cyst data, characteristics, but also all the socioeconomic characteristics of the patients for research, et cetera. So the quality has already improved just based on modernizing the program. You bring in a dramatic amount of patients. Across this country, around 17 to 19 percent of MRIs of the abdomen will identify a pancreatic cyst run 3% of CAT scans. That is a significant number of patients that are then coming into your practice, and I think it really builds out a robust program. And then once those patients are in surveillance, yes, the majority of them in the pancreatic world will need serial lifelong MRI imaging. So that really drives revenue on the radiology side. A significant portion of them will need endoscopic ultrasound assessment with an interventional gastroenterologist. And around 5% of patients, in my opinion, in the pancreatic cyst world will end up getting a pancreatic surgery. For administrators, you know better than I, one pancreatic operation, whether it’s a Whipple, a central pancreatectomy, or a distal pancreatectomy, or a total pancreatectomy, brings significant revenue to an institution. It is a high reimbursable case, so really one pancreas case could pay for this program a year. And we really did see that in live time.
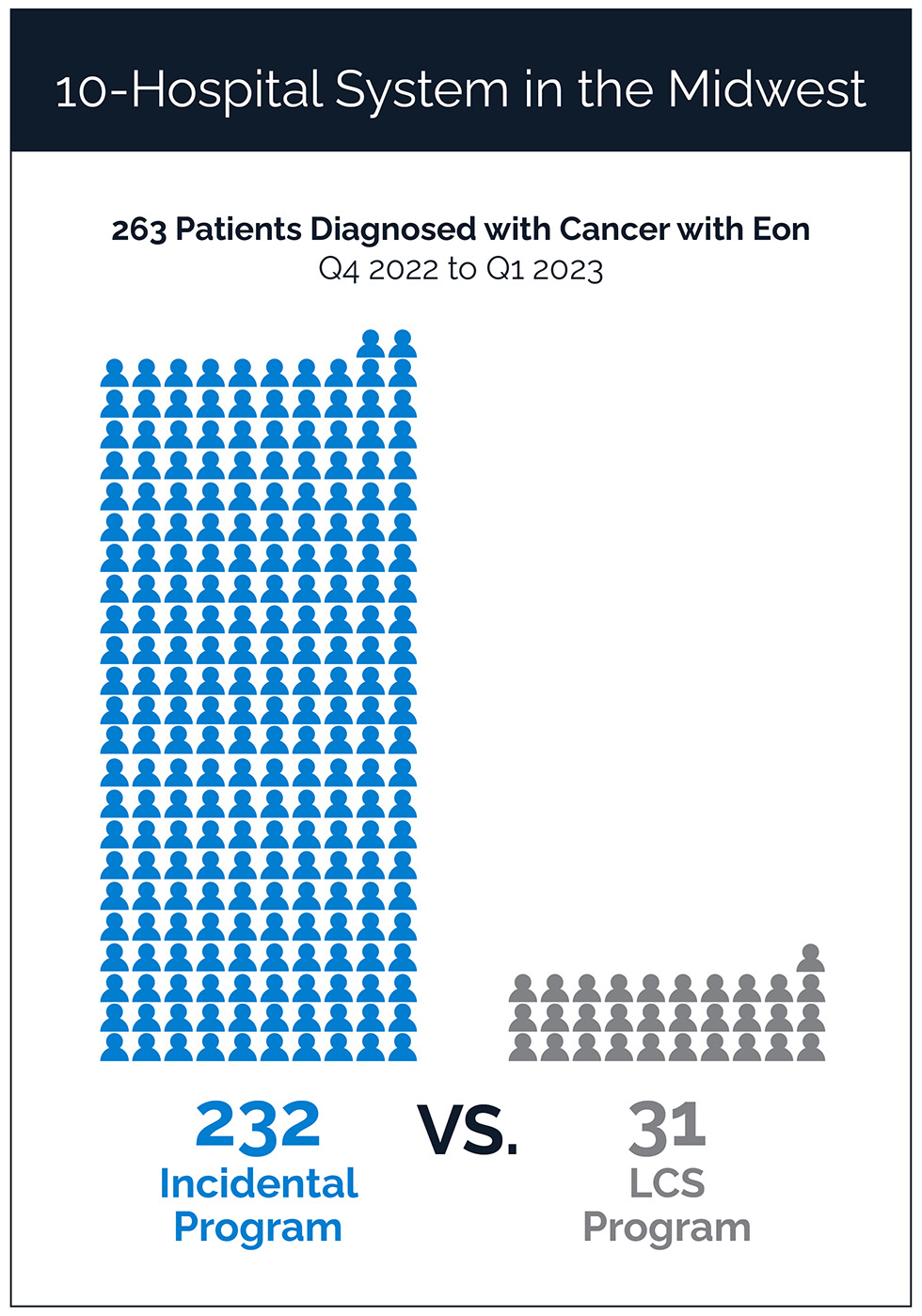
Gieske, MD: We’ve done over 36,000 screens since we started and that’s allowed us that increase in business to hire additional staff, and our third dedicated thoracic surgeon. And because as we’ve grown our lung cancer screening program we’ve also grown our incidental pulmonary program, which we started about the same time. We hired Eon in late 2021 and our incidental program has grown substantially since that time. We knew there was gonna be a wave and an impact, and that’s why we hired a third dedicated nurse navigator at the time, but we really could use a fourth. The other cool thing that we’ve done because of this increased revenue, increased business, we’ve been able to hire a team of outreach specialists. We have a team of 12 nurses that are outreach specialists that contact any patient with an outstanding lung cancer screening order that hasn’t had an appointment made within 72 hours. They also reach out to outstanding cologuards and outstanding mammograms. And that’s really been highly impactful for the uptake in the business program.
Bridget LeGrazie: We see the lung cancer screening and the incidental findings as a funnel into the cancer program. And as we’ve expanded the program across our sites, we’ve seen our numbers grow. One of the really important things is just getting right to the bottom of patient retention numbers. So if we start them and we’re nurturing that relationship, whether they go on to have a cancer diagnosis now or later, are they staying with us once they get to the cancer diagnosis point for the rest of their care?
What we’ve found is a 90% retention rate within the program for patients that are diagnosed. And those are patients that are going on for surgery, they’re going on for radiation therapy, they’re going on for infusion therapy. And we’re really proud of that. That in itself, as you said, pays for the program.
The transformative impact of AI on oncology care is clear from the success stories shared above. All four oncology leaders agree that placing the responsibility squarely within their service lines has helped streamline care coordination and facilitate early cancer detection, leading to both clinical and financial success. As we look to the future of oncology, it’s clear that AI will continue to play a prominent role in how we diagnose, manage and care for patients.
Interested in understanding the clinical and financial impact of a comprehensive cancer screening and incidental program at your facility? Contact us for your custom opportunity assessment.
Click to learn more about how leading health systems built their lung screening and incidentals program and the results they are seeing.